
These and related results strongly indicate that the bivalent antibodies produce an aggregation of the surface immunoglobulin molecules in the plane of the membrane, which can occur only if the immunoglobulin molecules are free to diffuse in the membrane. These effects do not occur, however, if the bivalent antibodies are replaced by their univalent Fab fragments or if the antibody experiments are carried out at 0 degrees C instead of 37 degrees C.
The antibodies induce a redistribution and pinocytosis of these surface immunoglobulins, so that within about 30 minutes at 37 degrees C the surface immunoglobulins are completely swept out of the membrane. (70) showing the remarkable effects produced on lymphocytes by the addition of antibodies directed to their surface immunoglobulin molecules. T-here has also appeared a study by Taylor et al. Note added in proof: Since this article was written, we have obtained electron microscopic evidence (69) that the concanavalin A binding sites on the membranes of SV40 virus-transformed mouse fibroblasts (3T3 cells) are more clustered than the sites on the membranes of normal cells, as predicted by the hypothesis represented in Fig. As examples, experimentally testable mechanisms are suggested for cell surface changes in malignant transformation, and for cooperative effects exhibited in the interactions of membranes with some specific ligands. It therefore seems appropriate to suggest possible mechanisms for various membrane functions and membrane-mediated phenomena in the light of the model. Recent experiments with a wide variety of techniqes and several different membrane systems are described, all of which abet consistent with, and add much detail to, the fluid mosaic model.

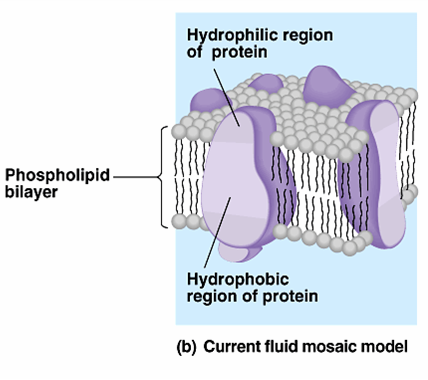
The fluid mosaic structure is therefore formally analogous to a two-dimensional oriented solution of integral proteins (or lipoproteins) in the viscous phospholipid bilayer solvent. The bulk of the phospholipid is organized as a discontinuous, fluid bilayer, although a small fraction of the lipid may interact specifically with the membrane proteins. These globular molecules are partially embedded in a matrix of phospholipid. In this model, the proteins that are integral to the membrane are a heterogeneous set of globular molecules, each arranged in an amphipathic structure, that is, with the ionic and highly polar groups protruding from the membrane into the aqueous phase, and the nonpolar groups largely buried in the hydrophobic interior of the membrane.
The model is consistent with the restrictions imposed by thermodynamics. A fluid mosaic model is presented for the gross organization and structure of the proteins and lipids of biological membranes.


 0 kommentar(er)
0 kommentar(er)
